*As of October 2024 in the U.S.
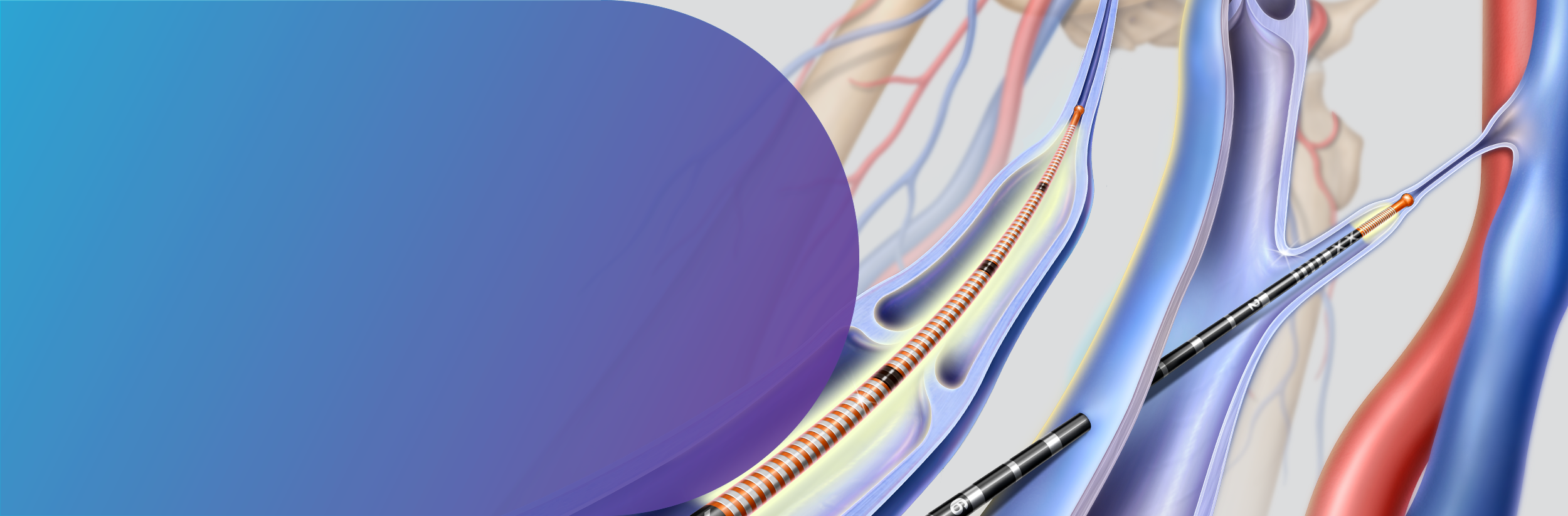
The Venclose™ EVSRF Catheter is intended for endovascular coagulation of blood vessels in patients with superficial vein reflux. The Venclose™ EVSRF Catheter is contraindicated in patients with thrombus in the vein segment to be treated. The Venclose Maven™ Catheter is intended for endovascular coagulation of blood vessels in patients with perforator and tributary vein reflux. The Venclose Maven™ Catheter is contraindicated in patients with thrombus in the vein segment to be treated.
Potential adverse events include but are not limited to: vessel perforation; skin discoloration; nerve injury; temporary paresthesia; thrombosis; deep vein thrombosis; phlebitis; hematoma; infection; skin burn; pulmonary embolism; and pain.
Please consult product labels and instructions for use for indications, contraindications, hazards, warnings, and precautions.
BD, the BD logo, digiRF, Venclose, and Venclose Maven are trademarks of Becton, Dickinson and Company or its affiliates. © 2025 BD. All Rights Reserved. © Illustrations by Mike Austin.